What is atmosphere?
The term “atmosphere” originates from Ancient Greek, where “atmós” signifies “vapour” or “steam,” and “sphaîra” refers to “sphere.” Today when we use this word we actually mean: a layer/layers of gas/gasses, enveloping a planet, held in place by the gravity of that planet. The atmosphere is a crucial aspect of our planet, playing a vital role in regulating temperature, weather patterns, and air quality. Atmosphere of Earth is a delicate balance of gases, composed mainly of nitrogen (78%) and oxygen (21%), as well as trace amounts of other gases such as carbon dioxide, argon, and neon. In this article, we will explore our atmosphere in detail. We will discuss its composition, structure, and functioning.
Some important characteristics of the atmosphere are:

- It is a mixture of different gases
- It regulates Insolation
- 99% of the atmospheric mass is within 32 km
- When the Earth is scaled down to the size of an apple, surprisingly its atmosphere is as thin as the apple’s peel.
- Atmosphere is fluid
- Has a base (surface) but no top
- High oxygen content and low carbon dioxide content
- The right amount of Greenhouse gases makes the surface temperature livable

Why is the atmosphere blue?
One word answer is ‘Rayleigh Scattering’
Sunlight reaches Earth’s atmosphere and is scattered in all directions by all the gases and particles in the air. Blue light is scattered more than the other colours because it travels in shorter, smaller waves. This is why we see a blue sky most of the time.
spaceplace.nasa.gov
What is atmospheric science?
Atmospheric science is the study of the Earth’s atmosphere and the processes that occur within it. This field encompasses a wide range of topics, including meteorology (the study of weather and climate), atmospheric chemistry, atmospheric physics, and atmospheric dynamics. Researchers in atmospheric science use a variety of tools and techniques. Chiefly, computer models, satellites, and ground-based observations, in order to study the atmosphere and predict weather patterns and climate change.
Why does our atmosphere exist?
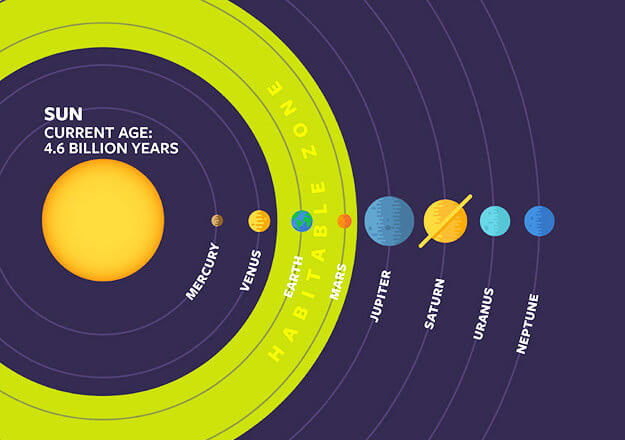
Located within the “Goldilocks Zone,” Earth resides in an environment that allows for liquid water to exist on its surface. The Goldilocks Zone refers to the habitable region around a star, that is where temperatures are neither too hot nor too cold for liquid water. Earth’s average distance from the Sun, which is 149,600,000 kilometres (92,900,000 miles) and referred to as 1 Astronomical Unit (AU), places it in the ideal location within this zone.
Role of Earth’s Atmosphere
Atmosphere is essential for all the life existing on Earth. Organisms require various gases, including oxygen, carbon dioxide, and nitrogen present in our atmosphere. Ozone (O3) composed of three oxygen atoms which is present in the upper atmosphere absorbs harmful ultraviolet (UV) radiations. It acts as a protective shield, giving all the life forms the prerequisites to sustain and flourish. By blocking the harmful radiations of the sun during the day and by not letting the warmth of the earth escape at night with the help of Greenhouse gases, the atmosphere regulates the required temperature for all the biophysical processes. The hydrological cycle, which operates in the lower limits of the atmosphere is responsible for all the freshwater movement. Some of the important functions of the atmosphere are summarised below:
- Protection from Solar Radiation: The atmosphere acts as a shield that absorbs and scatters harmful solar radiation, including ultraviolet (UV) rays. This protection is vital for the survival of living organisms.
- Regulation of Temperature: The atmosphere helps regulate the Earth’s temperature by absorbing and redistributing solar energy. It traps heat close to the Earth’s surface, creating a greenhouse effect that maintains a relatively stable and habitable temperature range.
- Weather and Climate: The atmosphere is the key driver of weather and climate patterns. It governs the movement of air masses, the formation of clouds, precipitation, and wind patterns, all of which influence climate at both local and global scales.
- Circulation and Weather Systems: The atmosphere’s circulation patterns, driven by uneven heating and the rotation of the Earth, lead to the formation of high-pressure and low-pressure systems, which in turn produce weather events such as rain, storms, and winds.
- Distribution of Moisture: The atmosphere facilitates the movement of water vapour through the hydrological cycle, including evaporation, condensation, and precipitation. This distribution of moisture is crucial for maintaining ecosystems and providing freshwater resources.
- Cycling water and nutrients: The atmosphere plays a key role in the water cycle, helping to distribute water and nutrients around the earth.
- Respiration for Living Organisms: Plants and animals depend on the atmosphere for oxygen, which is essential for cellular respiration—a process that generates energy for life-sustaining functions.
- Gas Exchange: The atmosphere enables the exchange of gases (e.g., oxygen, carbon dioxide) between living organisms and the environment. Plants absorb carbon dioxide and release oxygen through photosynthesis, while animals breathe in oxygen and release carbon dioxide.
- Sound and Communication: The atmosphere allows for the transmission of sound waves, enabling communication and various activities of organisms.
- Aid in Navigation: The atmosphere aids navigation by allowing the use of celestial bodies for orientation, supporting various methods of transportation, and facilitating aviation.
Composition of atmosphere
When we casually use the word air, we essentially mean a mixture of gases. Therefore, our atmosphere which consists of air is in fact a mixture of many gases. Air consists of gases like nitrogen, oxygen, carbon dioxide, etc. Table 1 shows the composition of dry air.
| Gas Name | Chemical Formula | Percent Volume |
| Nitrogen | N2 | 78.08% |
| Oxygen | O2 | 20.95% |
| *Water | H2O | 0 to 4% |
| Argon | Ar | 0.93% |
| *Carbon Dioxide | CO2 | 0.0360% |
| Neon | Ne | 0.0018% |
| Helium | He | 0.0005% |
| *Methane | CH4 | 0.00017% |
| Hydrogen | H2 | 0.00005% |
| *Nitrous Oxide | N2O | 0.00003% |
| *Ozone | O3 | 0.000004% |
When we say “atmosphere” we refer to the envelope of air that surrounds the earth and other than these gases two more important constituents of the atmosphere are water vapour and aerosols.
>Water vapour: It varies with time and space and is an important atmospheric constituent.
>Aerosols: These are solid particles of sand, sea salt, dust, pollen grains, soot.etc., suspended in the atmosphere.
If the water vapour and aerosols are not considered then observations show that the proportion of gases is constant and thus dry air will be stable up to the height of 80 km. The atmosphere is held close to earth due to its gravitational pull, this is the reason it is densest near the surface and rarefies as we rise up.
In the higher layers of the atmosphere, not only density but the composition of gases also vary, so much so that at the height of 120 km oxygen is almost negligible whereas CO2 and water vapour are found only up to 90 km above sea level.
Gasses of our atmosphere:
Oxygen:
It is the most important and second most abundant ( 21% by volume ) gas in the atmosphere. All organisms need oxygen for metabolic functions of the body to produce energy from food, for the release of energy, and for the release of carbon dioxide as a by-product. Oxygen is also required for the process of combustion. Besides oxygen in combination with other elements forms oxides.
Nitrogen:
78% by volume of our total atmosphere is nitrogen. A comparatively inert gas that forms the basis of the ‘Nitrogen Cycle’. Nitrogen is made available to living organisms through fixation and is required by the most important processes of our body as it is the major part of amino acids which form protein, DNA…etc. Plants get nitrogen through the soil. Nitrogen dilutes oxygen which helps in controlling combustion.
Carbon Dioxide:
0.03% by volume this is another important gas in our atmosphere. Carbon dioxide ( CO2) is a ‘Greenhouse Gas’ and is a product of the combustion of carbon-based material, photosynthesis, and respiration. Due to its heat-absorbing capability, it contributes significantly to the heat budget, having a major effect on the world climate and global climate change.
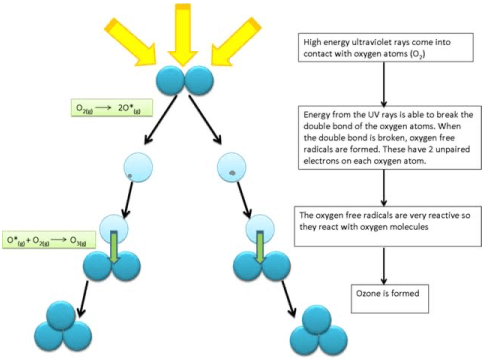
Ozone:
Ozone or O3 is concentrated mainly between 15 to 35 km. In the upper layers of the atmosphere, the Oxygen i.e. O2 molecule is broken by the ultraviolet radiation of the sun.
O2 = O + O
These free oxygen atoms combine with the O2 molecule to form ozone (O3)

This O3 molecule itself is unstable and is affected by ozone-depleting substances (ODS) like chlorofluorocarbons (CFCs) entering the upper atmosphere due to human activities and is the main cause of ozone depletion.
The ozone layer forms less than 0.00005% of the atmosphere but is crucial in blocking the deadly ultraviolet radiations from the sun.

The figure on the left shows an Ozone hole over the Antarctic. It is a zone of extremely depleted ozone gas.
What is the Montreal Protocol?
Water Vapour:
The content of water vapour in the atmosphere is closely related to the temperature of air and therefore is highly variable with latitude and altitude, but there are exceptions to this generalisation. Usually, in low latitudes, the humidity will be highest in summers but the same may not hold true if humidity is observed at a deserted place. Water vapour is variable from 0.02% in cold dry air to 4% in hot and humid air by volume. Water vapour is important for precipitation and cloud formation.
Aerosols:
Minute particles suspended in the atmosphere are known as aerosols. If these particles are sufficiently large they scatter and absorb the sunlight, resulting in reduced visibility.
Aerosols enter the atmosphere through natural as well as human activities. Volcanic activity, wind-raised dust, sea sprays, forest fires, pollution, agricultural practices, etc are some of its sources.
Aerosols affect the atmosphere by reflecting back sun’s radiations back into space. They also form the cloud condensation nuclei around which the cloud formation occurs.
When artificial chemical aerosols (CFCs found in spray cans and refrigerants) reach the stratosphere, usually during the winter months and particularly in the polar regions due to a phenomenon known as the ‘Polar Vortex’. They react with stratospheric ozone causing ‘Ozone depletion’.
What determines the composition of the Atmosphere?

A planet’s escape velocity plays a role in determining the composition and retention of its atmosphere. Escape velocity is the minimum speed an object (such as a gas molecule) needs to reach to break free from a celestial body’s gravitational pull and escape into space. The escape velocity is influenced by the mass and radius of the planet…Continue reading
What are Permanent and Variable Gases
| Permanent Gases | Variable gases |
| Nitrogen | Water Vapour |
| Oxygen | Carbon Dioxide |
| Argon | Methane |
| Neon | Nitrous Oxide |
| Helium | Ozone |
| Hydrogen | Particles |
| Xenon | Chlorofluorocarbons |
Permanent or fixed gases are those gases that do not vary with time in our lifetime. However, they may vary in geological time or in billions of years.
Variable gases on the other hand change constantly even with the season. Such gases in fact may have an impact on the climate and weather of local areas.
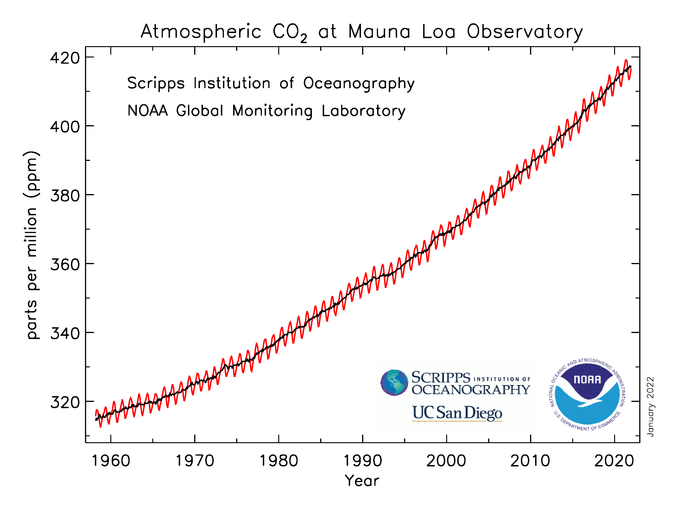
The atmospheric concentration of CO2 has increased from 280 ppm in 1750 to 413 ppm in 2020.
Atmospheric Cycles & Residence Time of Gases

The gases in our atmosphere keep changing. As we read in the above section, not all gases are constant, throughout the geologic history of our planet, the composition of these gases has changed. But in our lifetime some gases fluctuate at a faster pace, while others may take billions of years. Such fluctuations affect the ecosystems as every gas is not only a product but also a contributor to the biotic and abiotic cycle.

Since every gas goes through a certain process, each gas takes time to get absorbed either by an organism or by a geologic process. This is known as the residence time of gas. Human activities have disrupted the natural carbon cycle, resulting in unusual levels of carbon dioxide in the atmosphere. This anthropogenic influence has led to carbon dioxide being classified as a variable gas.
Greenhouse gas, 386 ppm, Residence time is 300-1000 Years

What are Atmospheric State Variables?
Answer: Pressure, Temperature & Density
Atmospheric state variables are related to one another by Ideal Gas Law(IDL).
Ideal Gas Law states that the pressure, temperature & volume of a gas are related to each other.
IDL is also referred to as the “Equation of State”
Vertical Structure of Atmosphere
Since we move on the surface of the earth horizontally almost all the time, we practically miss the vertical variation of our atmosphere. But when we study this vertical structure we understand the uniqueness of our atmosphere. Due to the layered vertical structure of the atmosphere, Sun’s harmful radiations are blocked and the right temperature is maintained. Our atmosphere mainly consists of 5 layers. Each layer has different properties and is divided by a “pause”. The word pause literally means a stagnancy in temperature, beyond which the characteristics change again.
Layers of Atmosphere
Troposphere
The word “tropos” in troposphere comes from the Greek word “τροπος”, which means “turn” or “change”. This is because the troposphere is the layer of the atmosphere where the air is constantly mixing and changing. The troposphere is also where most weather phenomena occur, such as clouds, precipitation, and storms.
The term “troposphere” was first introduced by the French meteorologist Léon Teisserenc de Bort in 1908. He used the term to describe the lowest layer of the atmosphere, which is characterized by its turbulent air and changing weather conditions.
The Troposphere extends from the surface up to an altitude of 8 km at the poles and 18 km at the equator. It is thicker at the equator due to the rising of heated air to greater heights. The troposphere is bounded by the Tropopause and its temperature decreases with increasing altitude at a rate of 6.5°C per kilometre, reaching -45°C at the poles and -80°C over the equator at the Tropopause (with a greater fall in temperature above the equator due to its greater thickness). This decrease in temperature is known as the ‘normal lapse rate’.
The troposphere is characterized by a temperature inversion, turbulence, and eddies, and is meteorologically the most significant zone in the atmosphere, as almost all weather phenomena like rainfall, fog, hailstorms, etc. are confined to this layer. It is also known as the convective region as all convection stops at the Tropopause. The troposphere is the region where weather occurs, with all cyclones, anticyclones, storms, and precipitation taking place here because all water vapour and solid particles are present in this layer. It is influenced by seasons and jet streams.
Stratosphere

The word “stratos” in stratosphere comes from the Greek word “στρατος”, which means “army” or “layer”. This is because the stratosphere is a layer of the atmosphere that is characterized by its stable temperature and lack of turbulence.
The stratosphere is a layer of the Earth’s atmosphere that lies beyond the troposphere and extends up to an altitude of 50 km from the surface. It is characterized by a constant temperature for some distance, followed by a rise to 0°C at 50 km altitude, due to the presence of ozone which absorbs harmful ultraviolet radiation. The stratosphere is very dry and contains less water vapour compared to other layers of the atmosphere. This layer is almost cloud-free, making it ideal for flying aeroplanes. Occasionally, cirrus clouds can be found at lower levels in the stratosphere. Aeroplanes often fly in the lower stratosphere or in the upper troposphere to avoid turbulence as the weather conditions are calm at this altitude.
Mesosphere
The word “meso” in mesosphere comes from the Greek word “μέσος”, which means “middle”. This is because the mesosphere is the middle layer of the Earth’s atmosphere. It is located above the stratosphere and below the thermosphere.
The mesosphere is a layer of the Earth’s atmosphere that lies beyond the ozone layer and extends up to an altitude of 80 km from the surface. The temperature in the mesosphere gradually decreases to -100°C at an altitude of 80 km. This layer is also where meteorites burn up upon entering the Earth’s atmosphere from space.
Thermosphere
The word “thermos” comes from the Greek words “θερμός” (thermos), meaning “heat”. This is because the thermosphere is the hottest layer of the Earth’s atmosphere.
The thermosphere is characterized by a rapid increase in temperature with height. It encompasses the ionosphere, which lies between 80-400 km and aids in radio transmission by reflecting radio waves back to Earth. Despite the high temperature, the extremely low pressure in the thermosphere means that it does not feel warm. The International Space Station and Lower Earth Orbit (LEO) Satellites orbit in this layer, where the atmosphere is extremely thin with gas molecules spaced hundreds of kilometres apart, so objects or people do not experience the heat. Auroras can be observed in the lower parts of the thermosphere.
Exosphere
The word “exo” comes from the Greek words “έξω” (exo), meaning “outside”. This is because the exosphere is the outermost layer of the atmosphere, where the air particles are no longer bound to the Earth by gravity.
The exosphere extends from the top of the thermosphere to the edge of space. It is a very thin layer of air, and the particles are constantly escaping into space.
This layer is characterized by its very low density and high temperature. The temperature in the exosphere can reach up to 2,000 degrees Celsius (3,600 degrees Fahrenheit), but the air pressure is so low that it is almost nonexistent.
The exosphere plays an important role in protecting the Earth from the solar wind. The solar wind is a stream of charged particles that is emitted from the sun. The exosphere helps to deflect the solar wind and protect the Earth from its harmful effects.
Here are some of the key characteristics of the exosphere:
- It is the outermost layer of the Earth’s atmosphere.
- It is a very thin layer of air with a very low density.
- The temperature in the exosphere is very high, but the air pressure is extremely low.
- The exosphere plays an important role in protecting the Earth from the solar wind.
The exosphere is also a very mysterious region. Scientists are still learning about the exosphere and its role in protecting the Earth.



Surrender Singh

OCEANOGRAPHY By
Savindra Singh
Suggested books
